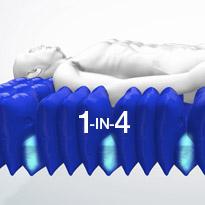
Active 1-in-4 cell cycle
The active 1-in-4 cell cycle supports 75% of the patient's body at all times, on groups of three inflated cells, whilst the fourth cell deflates sufficiently to redistribute the pressure and encourage tissue reperfusion.
Additional benefits include enhanced patient comfort, less awareness of support surface movement and a reduction of stimulus-related complications such as muscle spasm
TISSUEgardTM
TISSUEgard enables the partial immersion and envelopment of the patient into the support surface, reducing the pressure on the patient's skin and decreasing the pressure differential between inflated and deflated cells, which helps reduce tissue strain and associated shearing forces.
DEEP CELL THERAPY TM
DEEP CELL THERAPY allows the cells of the QUATTRO Acute to run at lower internal pressures. This minimises the pressure applied to the patient's skin and subcutaneous tissues during the cycle.
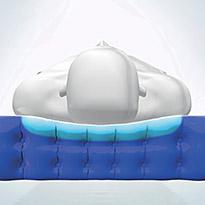
Ortho-differential SupportTM
ODS results in firmer outer edges of the mattress which facilitate patient transfers and provide extra support, safety and comfort for larger or heavier patients. It also creates a softer central area of the mattress, ideal for smaller, lighter patients.
Multi-stretch cover
The multi-stretch cover promotes tissue offloading during cell deflation and reduces shear and friction during patient movement or manual repositioning. The material is waterproof, moisture-vapour permeable and all seams are welded to protect the inside of the mattress from fluid ingress.
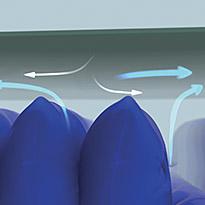
Microclimate management
The waterproof, moisture-vapour permeable properties of the multi-stretch cover, combined with a low-air-loss feature within the mattress, helps manage the skin 'microclimate' between the patient and support surface.

Fully launderable
QUATTRO Acute and Plus are fully launderable to help reduce the risk of infection and / or cross contamination to its lowest practical level.

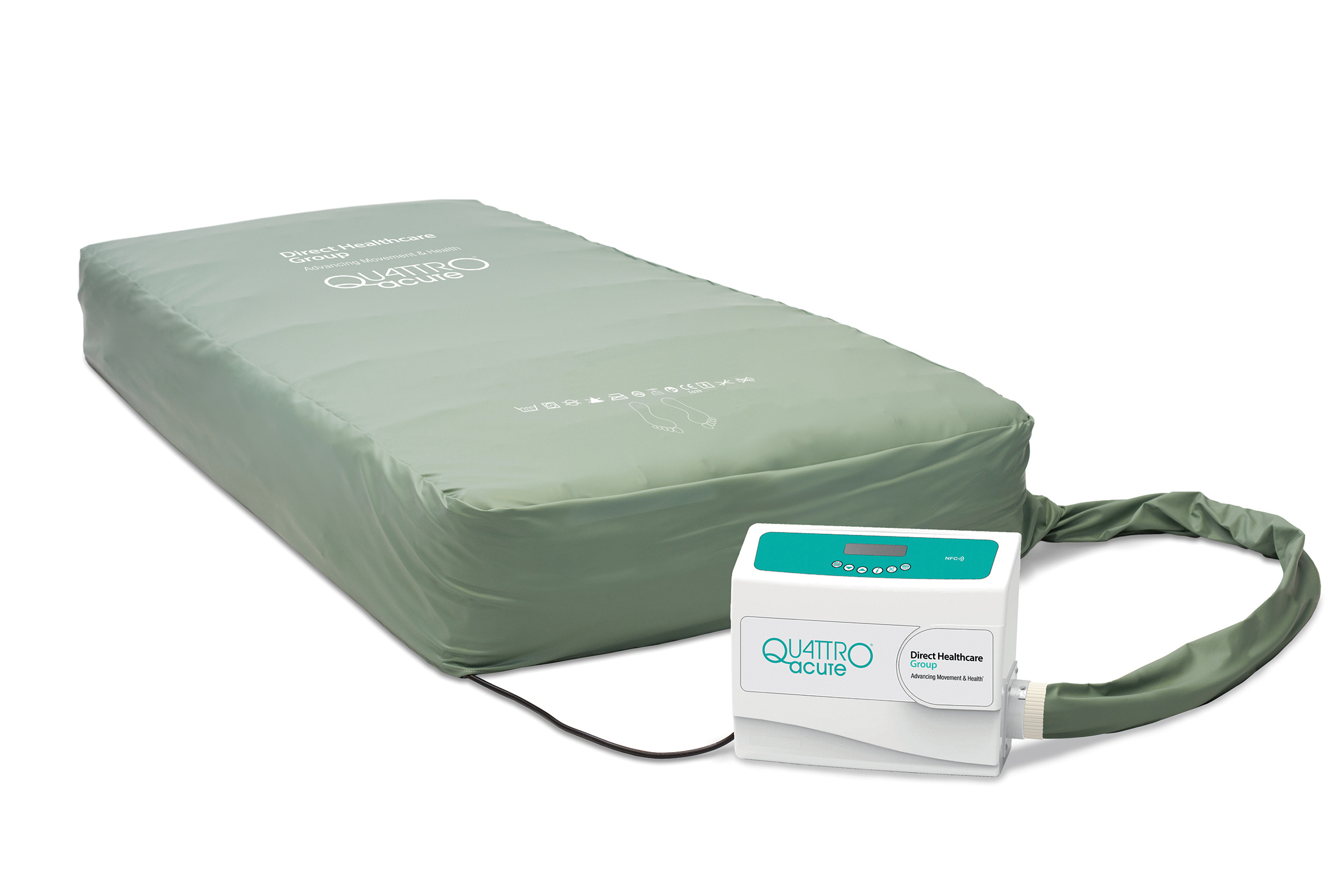
Simply designed interface
All QUATTRO® products are designed to be intuitive. The simply designed interface and easy-touch buttons allow the carer to control all essential functions such as the Dual therapy modes providing either Active therapy or Continuous low pressure, Soft, Medium or Firm comfort settings as well as Max-Inflate mode.

Fast, safe and easy manual interventions
Manual interventions such as CPR are fast, safe and easy to operate.

Patient Transport Facility
The Patient Transport Facility turns the mattress into a static unpowered support surface for up to 12 hours.





-A-DHG-Company-01-7-1614716979.png)

