
370kg weight limit
The mattress can support a patient weight of 370kg. The extra durable air cells are constructed from reinforced PU coated nylon for added strength and durability.
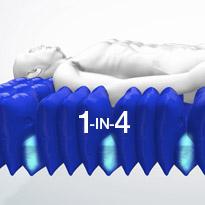
Active 1-in-4 cell cycle
The active 1-in-4 cell cycle supports 75% of the patient's body at all times, on groups of three inflated cells, whilst the fourth cell deflates sufficiently to redistribute the pressure and encourage tissue reperfusion. Additional benefits include enhanced patient comfort, less awareness of support surface movement and a reduction of stimulus-related complications such as muscle spasm.

Removable side bolsters
Ideal for use with width-retractable beds to allow easy movement of complete bed system with patient in-situ.
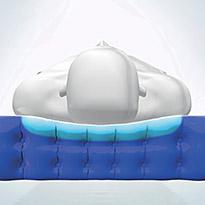
Ortho-differential support™
Cell design provides optimal comfort and support for varying body shapes and sizes.

Simply designed interface
All QUATTRO products are designed to be intuitive. The simply designed interface and easy-touch buttons allow the carer to control all essential functions such as the Dual therapy modes providing either Active therapy or Continuous low pressure, Soft, Medium or Firm comfort settings as well as Max-Inflate mode.

Multi-stretch cover
Multi-stretch cover is waterproof and moisture-vapour permeable with welded seams and can be removed for laundering
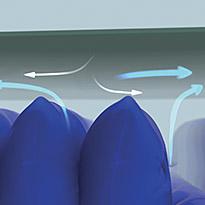
Microclimate
The waterproof, moisture-vapour permeable properties of the multi-stretch cover, combined with a low-air-loss feature within the mattress, helps manage the skin 'microclimate' between the patient and support surface.

Patient transport facility
The Patient Transport Facility turns the mattress into a static unpowered support surface for up to 12 hours.The Patient Transport Facility turns the mattress into a static unpowered support surface for up to 12 hours.


-A-DHG-Company-01-7-1614716979.png)

